Global Biosimilar Markets: Europe vs United States
 Feb, 16 2026
Feb, 16 2026
When you hear the word "generic," you probably think of cheap pills that do the same thing as brand-name drugs. But biosimilars aren’t like that. They’re not copies of a chemical pill. They’re copies of living, complex biologic medicines - drugs made from living cells, often used to treat cancer, autoimmune diseases, and diabetes. And when it comes to how these drugs are approved and used, Europe and the United States are playing two completely different games - even though they’re trying to reach the same finish line.
What Exactly Is a Biosimilar?
A biosimilar isn’t a generic. Generics are chemically identical to their brand-name counterparts. Biosimilars are highly similar - but not identical - to a reference biologic drug. Think of it like two handcrafted wooden chairs. They look the same, feel the same, and serve the same purpose. But one was made by a different craftsman using slightly different tools. That’s biosimilars: same function, same safety, same effectiveness, but made with a different biological process.
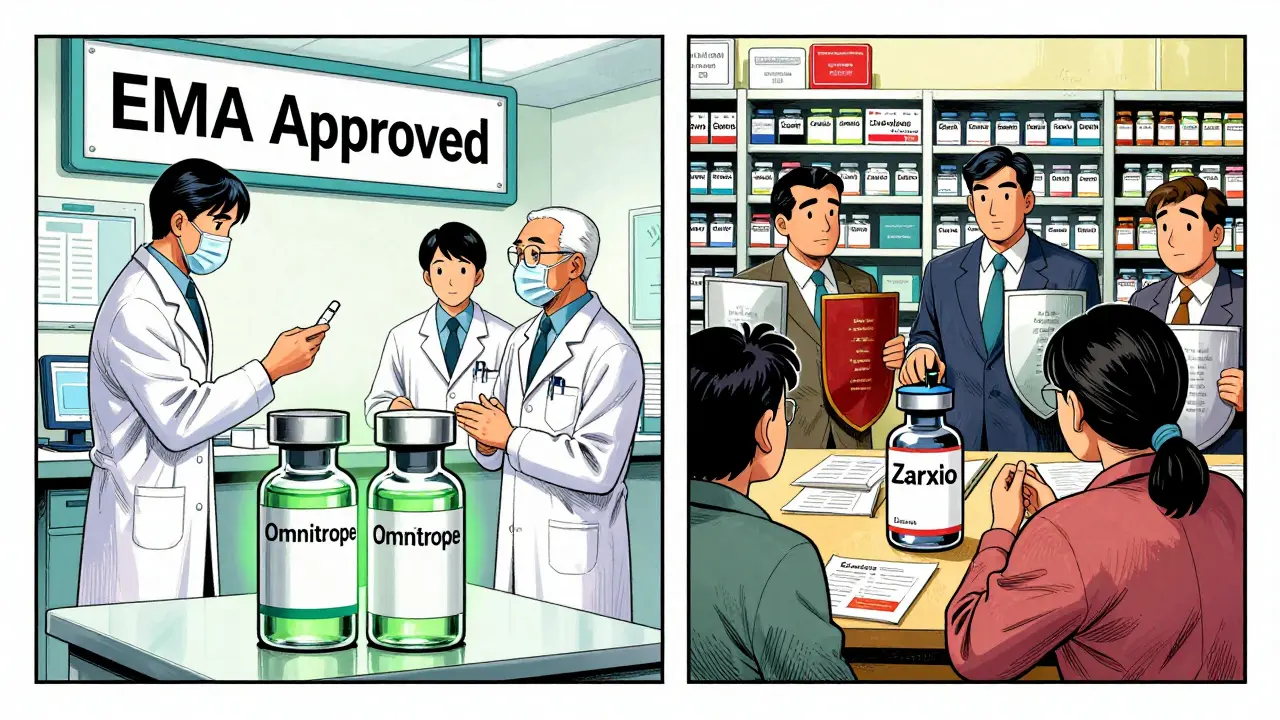
The first biosimilar ever approved was Omnitrope, a growth hormone, in Europe back in 2006. The European Medicines Agency (EMA) created the first clear rules for how to prove a biosimilar works. They didn’t demand a whole new set of clinical trials. Instead, they looked at the molecule’s structure, how it behaves in the lab, and a few targeted studies in patients. This "totality-of-evidence" approach saved time and money, and it worked. By 2024, Europe had approved over 100 biosimilars. The U.S. didn’t get its first until 2015 - Zarxio, a drug for low white blood cell counts. The delay wasn’t because the science was harder. It was because the system was slower.
Europe: The Pioneer with a Mature System
Europe didn’t just get there first. It built a system that made biosimilars easy to adopt. Hospitals in Germany, France, and the UK started using them almost as soon as they were approved. Why? Because the government and insurers pushed them. They ran tenders - competitive bidding - for drugs. If a biosimilar was 25% cheaper than the original, hospitals chose it. No debate. No paperwork. Just savings.
By 2024, Europe’s biosimilar market hit $13.16 billion in revenue, according to Precedence Research. That’s up from $8.9 billion in 2020. That’s a 13% annual growth rate. In some countries, biosimilars now make up over 80% of the market for drugs used to treat rheumatoid arthritis and Crohn’s disease. In oncology, they’re catching up fast. Sandoz, Fresenius Kabi, and Amgen dominate here. Germany, in particular, became a manufacturing hub. Companies from all over the world set up production there because the regulatory path was clear and the market was ready.
Doctors trust them. Patients accept them. Payers demand them. It’s a cycle: better regulation → faster adoption → lower prices → more use → more investment. Europe didn’t just allow biosimilars. It built an ecosystem around them.
United States: The Slow Starter, Now Accelerating
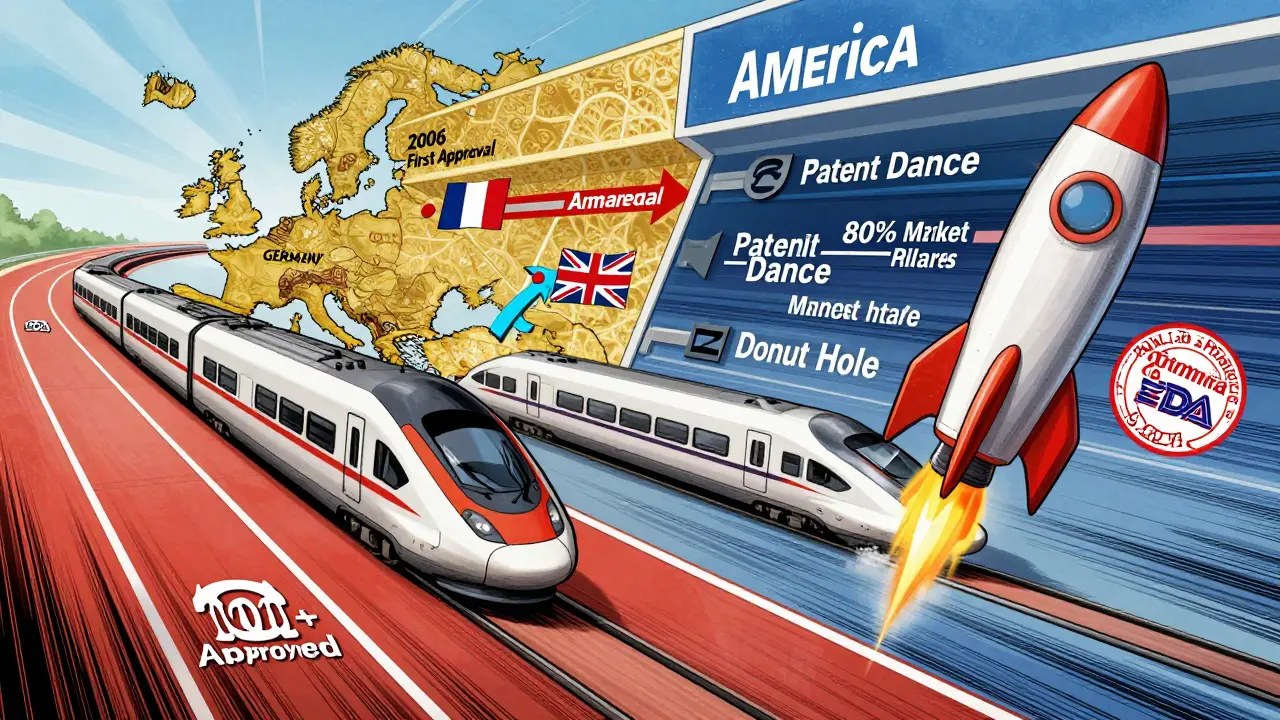
The U.S. had the same goal - lower drug costs - but the path was blocked. The Biologics Price Competition and Innovation Act (BPCIA) passed in 2009, but it came with a catch. It created a legal minefield called the "patent dance." Originator companies like AbbVie (maker of Humira) used every trick in the book: filing dozens of patents, dragging out lawsuits, and delaying biosimilar entry for years. Even when a biosimilar got FDA approval, it couldn’t hit the market for years because of legal battles.
By 2024, the U.S. had only approved 12 biosimilars - compared to over 100 in Europe. And many of those were for low-complexity drugs like filgrastim, used for infection prevention after chemotherapy. Complex drugs like Humira, Enbrel, and Remicade? Still mostly untouched by biosimilars.
But everything changed in 2024. The FDA dropped a bombshell: they no longer required switching studies to approve a biosimilar as "interchangeable." That meant a pharmacist could swap a biosimilar for the original drug without asking the doctor. In Europe, this had been standard for years. In the U.S., it was a huge barrier. Removing it was like taking down a toll booth on the highway to adoption.
Then came the Inflation Reduction Act of 2022. It eliminated the Medicare Part D coverage gap - the "donut hole" - and gave insurers a financial reason to push biosimilars. Why pay $10,000 for a biologic when a biosimilar costs $7,000 and the government helps cover the difference?
By 2024, the U.S. biosimilar market hit $10.9 billion. Projections show it could hit $30.2 billion by 2033. That’s an 18.5% annual growth rate - faster than Europe’s. Why? Because the U.S. has more high-value biologics coming off patent. Over 100 biologics will lose protection between 2025 and 2034. Humira alone represents a $232 billion opportunity. The market wasn’t slow because it didn’t want biosimilars. It was slow because the system was rigged. Now it’s unrigging.
Key Differences: Regulation, Adoption, and Price
Here’s the real contrast:
- Regulation: Europe’s EMA uses a streamlined, science-based process. The FDA used to demand extra clinical trials for interchangeability - now it doesn’t.
- Adoption: Europe’s centralized system means once a biosimilar is approved, it’s quickly added to hospital formularies. In the U.S., each hospital, insurer, and pharmacy decides independently - until now.
- Price: In Europe, biosimilars launch at 15-30% discounts. In the U.S., they’ve been priced higher - sometimes only 10% cheaper - because of patent delays and lack of competition. But with Humira biosimilars finally entering in 2023, prices are crashing. Some are now 70% cheaper than the original.
And here’s something surprising: Europe’s market is bigger today, but the U.S. is growing faster. North America (mostly the U.S.) is projected to overtake Europe in market size by 2027. Why? Because the U.S. has more money to spend on biologics. More patients. More high-cost drugs. More incentive to cut costs.

Who’s Winning?
It’s not a race with a winner. It’s two different paths to the same outcome: cheaper, effective drugs for millions.
Europe won the early game. They built the playbook. They proved biosimilars are safe. They showed doctors and patients it works. They created a culture of cost-conscious prescribing.
The U.S. is winning the next chapter. With Humira biosimilars now available, the dam has broken. Pfizer, Merck, and Samsung Bioepis are flooding the market. Insurers are pushing them. Patients are saving thousands. The FDA’s 2024 rule change is the turning point. The U.S. isn’t just catching up - it’s leapfrogging.
By 2030, biosimilars could save the U.S. healthcare system over $100 billion. In Europe, the savings are already in the tens of billions. Both markets are proving the same thing: when you make biosimilars easy to approve and easy to use, they don’t just compete - they transform.
What’s Next?
The future isn’t about Europe vs. the U.S. It’s about how fast biosimilars can reach patients. The next wave will be for even more complex drugs - like gene therapies and cell therapies. Manufacturing them is harder. Proving similarity is tougher. But the lessons from Europe and the U.S. are clear: regulation must be science-based, not legalistic. Payers must incentivize use. Doctors must be educated. Patients must be trusted.
The global biosimilars market is expected to hit $175 billion by 2034. That’s not just business. That’s better access. Lower costs. More treatment options. Whether it’s in a hospital in Berlin or a clinic in Chicago, the goal is the same: give patients the medicine they need without bankrupting them.
Are biosimilars the same as generics?
No. Generics are exact chemical copies of small-molecule drugs, like aspirin or metformin. Biosimilars are highly similar versions of complex biologic drugs made from living cells - like Humira or Enbrel. Because biologics are made from living organisms, biosimilars can’t be identical - but they must have no clinically meaningful differences in safety or effectiveness.
Why did Europe adopt biosimilars faster than the U.S.?
Europe created a clear, science-driven regulatory path in 2006 and tied it to hospital procurement policies. Once approved, biosimilars were automatically included in tender systems, and doctors were educated on their safety. In the U.S., patent litigation, lack of interchangeability rules, and fragmented payer systems slowed adoption. The FDA’s 2024 rule change removing switching studies is now helping close the gap.
Can a pharmacist substitute a biosimilar for the original drug without a doctor’s approval?
In Europe, yes - for interchangeable biosimilars. In the U.S., only if the biosimilar is designated as "interchangeable" by the FDA. Before June 2024, this was nearly impossible to achieve. Now, with the FDA dropping the switching study requirement, more biosimilars are expected to gain interchangeable status, allowing pharmacists to substitute them automatically.
Which countries in Europe lead in biosimilar use?
Germany, France, and the United Kingdom lead in adoption. Germany also leads in manufacturing, with several major biosimilar producers based there. These countries use hospital tenders and mandatory substitution policies to drive uptake, especially in oncology and autoimmune disease treatments.
Why is the U.S. market growing so fast now?
Three big reasons: the FDA removed the switching study barrier for interchangeable status, the Inflation Reduction Act gave insurers financial incentives to use biosimilars, and major biologics like Humira finally lost patent protection. With 14 Humira biosimilars approved and prices dropping by up to 70%, adoption is accelerating faster than ever.

Kancharla Pavan
February 16, 2026 AT 15:58Let me be crystal clear-Europe didn't "win" anything. They just had the luxury of a single-payer system that could bully hospitals into submission. In the U.S., we have competition, innovation, and patient choice. You can't just force doctors to switch drugs because some bureaucrat in Brussels said so. Biosimilars are great in theory, but when you remove the incentive for R&D by crushing profits, you kill the pipeline. The next breakthrough cancer drug won't come from a government-mandated tender. It'll come from a venture-backed biotech in Boston or San Diego. Europe's model is a dead end disguised as efficiency.
Liam Earney
February 16, 2026 AT 23:06Oh, I see… so Europe’s system is somehow… *better*? Because they have *tenders*? And *mandatory substitution*? And *government-controlled formularies*? How… quaint. I mean, really-when you strip away the patient’s autonomy, the doctor’s clinical judgment, and the market’s natural price signals… what’s left? A bureaucratic corpse with a nice spreadsheet. I’m not saying the U.S. hasn’t been a disaster-patent thickets, pharma lobbying, the whole circus-but at least here, a pharmacist doesn’t need a PhD in regulatory compliance just to fill a prescription. And I’m not even talking about the fact that in Germany, they’re now importing biosimilars from India because their own manufacturing can’t keep up. Oh, and the quality control? Ha. You ever read the EMA’s inspection reports? It’s like reading a horror novel written by a drunk audit clerk.
Oliver Calvert
February 17, 2026 AT 20:10The real story here is regulatory clarity. EMA got it right early: no need for redundant trials if the analytical data and pharmacokinetics match. FDA took 10 years to catch up. Now that they’ve dropped the interchangeability hurdle, adoption will explode. It’s not about Europe vs America-it’s about science vs bureaucracy. And the science is clear: biosimilars work. The rest is politics.
Sam Pearlman
February 19, 2026 AT 10:56Wait wait wait-so you’re telling me the U.S. is actually catching up? Like, *actually*? I thought we were still stuck in 2012 with Humira lawsuits dragging on forever. I mean, I’ve been on Enbrel for 8 years and my co-pay was $1,200 a month. Now my pharmacy just handed me a biosimilar for $300 and said "here you go, enjoy"? I’m not mad, I’m just… confused. Did we finally grow a spine? Or did the government just finally get tired of being owned by AbbVie? Either way, I’m not complaining. Pass the biosimilar.
Steph Carr
February 20, 2026 AT 10:52Europe didn’t "win"-they just didn’t let capitalism turn medicine into a hostage situation. In the U.S., we treat healthcare like a reality TV show where the prize is a $10,000 vial of Humira and the villain is anyone who dares to suggest a cheaper alternative. Meanwhile, in Germany, a nurse walks into the pharmacy, says "give me the biosimilar," and walks out with a smile. No drama. No patent dance. Just a human being getting the medicine they need. And honestly? That’s not socialism. That’s basic human decency. But sure, keep calling it "socialized medicine." It makes your outrage feel more righteous.
Brenda K. Wolfgram Moore
February 20, 2026 AT 20:33It’s not about who’s ahead. It’s about what works for patients. Europe showed us the path: clear rules, fair pricing, and trust in science. The U.S. spent 15 years playing whack-a-mole with patents instead of focusing on access. But now? The dam’s broken. Humira biosimilars at 70% off? That’s not a trend-that’s a revolution. And it’s happening because patients, insurers, and pharmacists finally said enough. This isn’t about ideology. It’s about cost. And when the cost drops, the right thing becomes the only thing.
Linda Franchock
February 22, 2026 AT 07:43Let’s be real-no one in the U.S. cared about biosimilars until their insurance started refusing to pay for the brand name. Suddenly, everyone’s an expert. "Oh, biosimilars are just as good!" Yeah, you think so now that your co-pay dropped from $1,500 to $200. Meanwhile, in Europe, they’ve been using them for 18 years and nobody’s dying. The difference? Europeans don’t wait for a crisis to fix a system. They fix it before it breaks. And honestly? That’s just… smarter.
Prateek Nalwaya
February 22, 2026 AT 09:53Isn’t it fascinating how two systems, born of different philosophies, converged on the same truth? Europe, with its collective ethos, built a pipeline of trust-regulation as a ladder, not a wall. America, with its chaotic individualism, stumbled through patent wars and corporate gatekeeping… until the market itself rebelled. Now, with Humira’s monopoly shattered, we’re witnessing the invisible hand of economics finally do what ethics and policy couldn’t. It’s not victory-it’s evolution. And perhaps, just perhaps, the next wave of biologics-gene therapies, CAR-Ts-will be built not on litigation, but on collaboration. The molecules don’t care where they’re made. They only care if they heal.
Agnes Miller
February 23, 2026 AT 08:38the fda thing in 2024 was a game changer honestly like i didnt even know they removed switching studies until my pharmacist told me my enbrel was now a biosimilar and i was like wait what? and then i looked it up and yeah they just said "eh its fine" like its 2024 and we finally got smart? also the inflation reduction act helped so much my mom got her rheumatoid arthritis med cut in half price like she cried. this is not politics. this is just… better.
Geoff Forbes
February 24, 2026 AT 11:55You think Europe’s system is better? They’re just copying American innovation and then undercutting it with state control. The FDA didn’t slow things down-it protected patients from subpar generics. Now that the U.S. is finally catching up, you want to hand it all over to German bureaucrats who think a biosimilar is a "substitute"? Newsflash: biologics aren’t aspirin. You don’t just swap them like socks. The EMA’s "totality of evidence" is a fancy way of saying "we didn’t do enough trials." And don’t get me started on the manufacturing standards in Eastern Europe. I’ve seen the reports. Half the biosimilars imported into the EU are made in places where the water isn’t even potable. But sure, keep drinking the Kool-Aid.
Jonathan Ruth
February 24, 2026 AT 23:43Europe’s "success" is built on a foundation of socialist control and price fixing. The U.S. system is broken? Maybe. But at least it’s not a government-run monopoly pretending to be a market. The FDA didn’t delay biosimilars-Big Pharma did. And now that the patents are finally falling, the real innovation is starting. The U.S. isn’t catching up. We’re just getting started. By 2030, we’ll be the undisputed leader in biosimilar manufacturing, R&D, and access. Because we don’t wait for permission. We build it.
guy greenfeld
February 26, 2026 AT 17:40Think about this: what if the entire biosimilar debate is a distraction? What if the real story is that pharmaceutical companies have been using biosimilars as a Trojan horse to extend monopolies under the guise of competition? The FDA’s 2024 rule change? A smoke screen. The Inflation Reduction Act? A political stunt to make voters feel good while the real cost-cutting happens in the shadows of Medicare negotiations. And Europe? They’ve been playing the same game since 2006-just with better PR. The truth? No one wants biosimilars to succeed. They just want to control who profits from them. The patients? They’re just the audience.
Adam Short
February 26, 2026 AT 20:30Europe? Pfft. They’re just using the U.S. as a testing ground for drug development, then swooping in with their socialist pricing to undercut us. We built the science. We funded the R&D. We bore the risk. And now they’re stealing our innovation with their state-run tenders? I don’t care if their biosimilars are 30% cheaper-if they’re made in a factory that doesn’t meet U.S. cGMP standards, they’re not safe. And don’t get me started on the fact that 60% of Europe’s biosimilars are manufactured by U.S. companies. We’re not losing-we’re being looted. And it’s about time someone called it out.
PRITAM BIJAPUR
February 27, 2026 AT 13:07It’s beautiful, really. Two paths-one rooted in collective wisdom, the other in chaotic innovation-both arriving at the same destination: patients getting life-saving drugs at a price they can afford. Europe didn’t just regulate; they cultivated trust. America didn’t just lag; it fought a war against its own inertia. And now? The tide is turning. The next frontier isn’t Europe vs America-it’s biosimilars vs greed. And for the first time, I feel like the patients might actually win. 🌱