Ledipasvir: How This Drug Changed Hepatitis C Treatment Forever
 Oct, 30 2025
Oct, 30 2025
Before ledipasvir, hepatitis C was a slow killer. People lived with it for years, sometimes decades, waiting for their liver to fail. Treatments were brutal-weekly injections, months of side effects like crushing fatigue and depression, and even then, success rates hovered around 50%. Then came ledipasvir. Not just another drug. A turning point.
What Ledipasvir Actually Does
Ledipasvir is not a standalone cure. It’s a direct-acting antiviral (DAA) that blocks a specific protein the hepatitis C virus needs to copy itself. It targets the NS5A protein, one of the virus’s key tools for replication. Without it, the virus can’t spread. But ledipasvir doesn’t work alone. It’s almost always paired with sofosbuvir, another DAA that shuts down a different part of the virus. Together, they form a one-pill, once-daily combo that’s been used by millions.
This combo-sofosbuvir/ledipasvir-was first approved by the FDA in 2014. By 2016, it was already clearing hepatitis C in over 95% of patients across all major genotypes. That’s not a slight improvement. That’s a revolution. For the first time, a cure was simple, safe, and effective for nearly everyone.
Why It Was a Game Changer
Old treatments used interferon and ribavirin. Interferon caused flu-like symptoms so bad many patients quit. Ribavirin could drop your red blood cell count so low you needed transfusions. Both lasted 24 to 48 weeks. Ledipasvir-based regimens? Twelve weeks. One pill. Fewer side effects than a common cold.
Real-world data from the UK’s NHS shows that after ledipasvir/sofosbuvir became widely available, hepatitis C cure rates jumped from 48% to 92% within three years. In prisons, where infection rates were high, cure rates hit 89%. In homeless populations, where adherence was a challenge, 85% still cleared the virus. That’s not theoretical. That’s lives saved.
Before ledipasvir, liver transplants for hepatitis C were common. After? Transplants dropped by 30% in countries with broad access to the drug. Why? Because people weren’t waiting until their livers failed. They were getting cured before it got that bad.
Who Benefits Most?
Ledipasvir/sofosbuvir works for genotype 1-the most common form of hepatitis C in the US, UK, and Europe. It also works for genotypes 4, 5, and 6. That covers over 90% of cases in Western countries. It’s effective whether you’ve had the virus for two years or twenty. It works whether you have cirrhosis or not. It works for people with HIV co-infection. It works for those who failed older treatments.
It’s not perfect for everyone. Genotype 3, which is more common in South Asia and parts of Eastern Europe, responds less reliably to ledipasvir alone. But even there, adding another drug like ribavirin or velpatasvir boosts success to 90%+. That’s why treatment is now personalized-not one-size-fits-all.

Side Effects? Almost None
Compared to what came before, ledipasvir’s side effect profile is almost unrecognizable. In clinical trials, the most common issues were mild fatigue and headache. Less than 2% of patients stopped treatment because of side effects. No one lost their hair. No one needed blood transfusions. No one was hospitalized because of the drug.
There’s one caveat: it can raise liver enzyme levels in people with advanced cirrhosis. That’s not because the drug is toxic-it’s because the liver is already damaged. Doctors monitor these levels, but they rarely require stopping treatment. The benefits still far outweigh the risks.
Cost and Access: The Real Barrier
When ledipasvir/sofosbuvir first hit the market, the price in the US was $94,500 for a 12-week course. That sparked global outrage. But the story didn’t end there.
Generic versions started appearing in 2017, especially from Indian manufacturers approved by the WHO. By 2020, the same treatment cost under $200 in many low- and middle-income countries. The UK’s NHS negotiated bulk deals and now offers it free at point of care. In some clinics, patients walk in, get tested, and leave with a prescription-no co-pay, no waiting.
Access is still uneven. In parts of Africa and Central Asia, the drug is still hard to find. But the model is proven: if you make it affordable, people get cured. And curing hepatitis C stops transmission. It breaks the chain.
What Happens After Treatment?
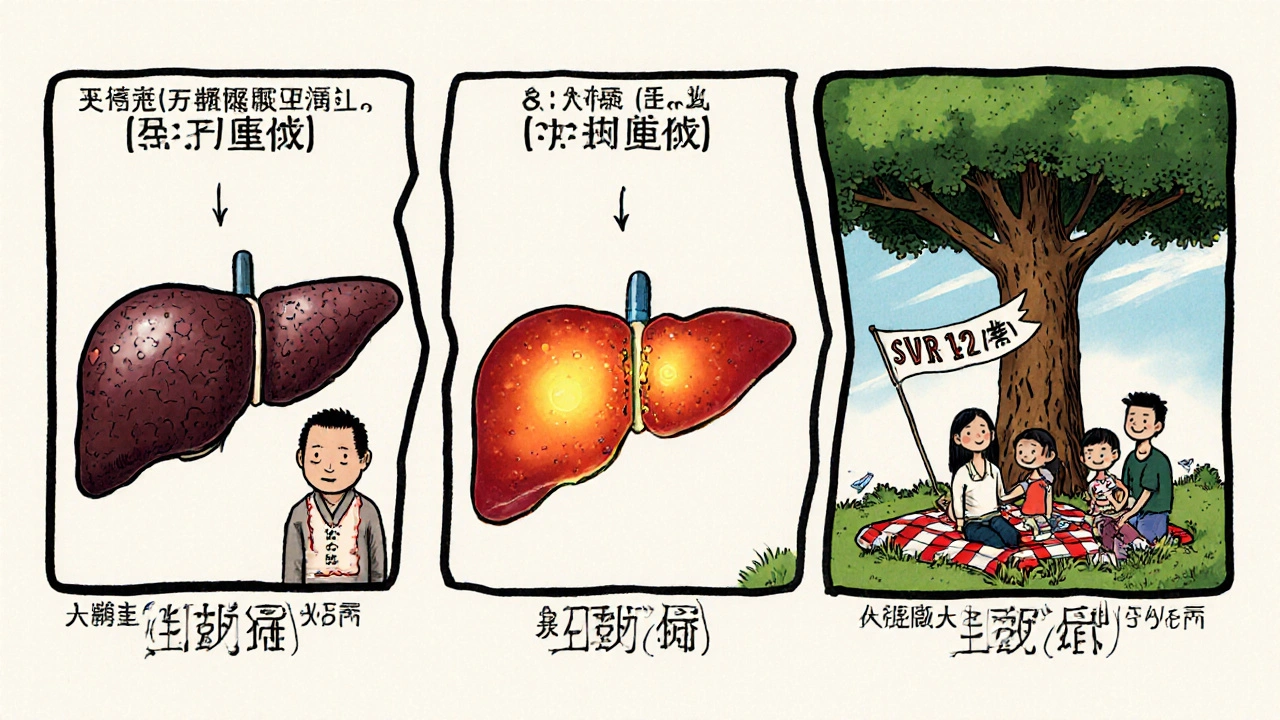
Success isn’t just about clearing the virus during treatment. It’s about staying cleared. After finishing ledipasvir/sofosbuvir, patients are tested again 12 weeks later. If the virus is undetectable, they’re considered cured. That’s called SVR12-sustained virologic response at 12 weeks.
Studies tracking patients for over 10 years show that once you hit SVR12, the chance of the virus coming back is less than 1%. Liver damage begins to reverse. Scarring improves. The risk of liver cancer drops by 70%. For people with cirrhosis, the risk of liver failure drops by 80%.
That’s not just medical jargon. That’s someone who no longer needs monthly doctor visits. Someone who can get life insurance. Someone who can plan for a future.
Where It Stands Today
Ledipasvir isn’t the newest drug anymore. Newer combinations like glecaprevir/pibrentasvir are now first-line in some places because they work faster and cover even more genotypes. But ledipasvir’s legacy is undeniable. It proved that hepatitis C could be cured with pills, not poison. It forced drug companies to rethink pricing. It gave public health systems a clear, scalable blueprint.
Today, the WHO aims to eliminate hepatitis C as a public health threat by 2030. Ledipasvir was the spark. It showed that elimination wasn’t a dream-it was a logistics problem. And logistics can be solved.
What You Need to Know If You’re Being Treated
- Take your pills every day, no matter how you feel. Missing doses can lead to resistance.
- Don’t take herbal supplements like St. John’s wort-they can interfere with the drug.
- Alcohol won’t stop the drug from working, but it will keep damaging your liver. Stop drinking.
- Get tested for HIV and hepatitis B before starting. Co-infections change the treatment plan.
- After treatment, follow up with your doctor for the SVR12 test. Don’t assume you’re cured just because you finished the pills.
There’s no stigma in having hepatitis C. It’s not about lifestyle choices. It’s about a virus that slipped through the cracks decades ago. Ledipasvir didn’t just treat it. It gave people back their lives.
Is ledipasvir still used today?
Yes, ledipasvir is still widely used, especially in combination with sofosbuvir. While newer drugs exist, this combo remains a first-line treatment in many countries due to its proven effectiveness, low side effect profile, and affordability through generics. It’s particularly common in regions where cost is a major factor.
Can ledipasvir cure hepatitis C in people with cirrhosis?
Absolutely. Ledipasvir/sofosbuvir is effective in people with compensated cirrhosis-meaning the liver is scarred but still functioning. Cure rates are slightly lower than in healthy livers (around 90-93%), but still far better than any previous treatment. For decompensated cirrhosis, doctors may add ribavirin or switch to a different combo, but ledipasvir still plays a key role.
How long does it take for ledipasvir to work?
The virus starts dropping within days of starting treatment. Most patients see viral levels fall below detectable limits within 4 weeks. But treatment lasts 12 weeks to ensure every last bit of the virus is gone. Stopping early increases the risk of the virus returning.
Does ledipasvir interact with other medications?
Yes. Ledipasvir can interact with certain heart medications (like amiodarone), antacids, and some seizure drugs. It also interacts with St. John’s wort and some HIV medications. Always tell your doctor what else you’re taking-even over-the-counter supplements. Most interactions can be managed with timing or dose changes.
Is ledipasvir safe during pregnancy?
Ledipasvir has not been studied extensively in pregnant women. Because it’s always used with sofosbuvir, and sofosbuvir has limited data in pregnancy, doctors usually delay treatment until after childbirth. However, curing hepatitis C before pregnancy is strongly recommended to prevent passing the virus to the baby.
Can you get hepatitis C again after being cured with ledipasvir?
Yes. Being cured doesn’t make you immune. You can be reinfected if you’re exposed again-through shared needles, unsterile tattoos, or unprotected sex with someone who has the virus. That’s why harm reduction and regular testing remain important, even after cure.

Anthony Griek
October 30, 2025 AT 15:28Man I remember when my uncle got diagnosed back in 2012. He was on interferon for a year and looked like he’d been through a war. Lost 40 pounds, couldn’t get out of bed. When they told him about this new pill combo? He cried. Didn’t even believe it at first. Now he’s hiking in Colorado every weekend. No transplant. No hospital visits. Just life.
Norman Rexford
October 30, 2025 AT 18:46sofosbuvir/ledipasvir is literally the reason america still has a functioning healthcare system lol. those pharma bros tried to charge 100k for a cure but we out here making generics cheaper than a pizza. america wins again. also why is everyone still talking about this like its new? its been 10 years bro
Wayne Keller
October 31, 2025 AT 10:33Just want to say this treatment changed everything. My sister had cirrhosis and was told she’d need a transplant. Twelve weeks on the pill, no side effects, and now her liver scans look normal. No one talks about this enough. It’s not magic. It’s science. And it works.
Shana Labed
October 31, 2025 AT 20:08OMG I just got chills reading this. Like… this is the kind of thing that makes you believe in humanity again. One pill. One month. One chance to live. And it’s not even expensive anymore. I want to hug every scientist who made this happen. 🙌❤️
California Daughter
November 2, 2025 AT 06:09Wait… so you’re saying… a drug that costs $200… can cure a disease that used to cost millions in transplants… and no one’s making a movie about this? That’s literally the plot of every superhero film ever… but real life? Nah. We’re too busy arguing about TikTok trends.
Vishwajeet Gade
November 3, 2025 AT 03:34India made the generic version. We didn't wait for america to be nice. We just did it. Now africa and southeast asia are getting cured because we refused to let pharma control life. This is not charity. This is justice.
Casey Crowell
November 4, 2025 AT 17:46This is the most beautiful thing I’ve read all year. 🥹 One pill. One cure. No more needles. No more suffering. My cousin was a junkie and got HCV from a dirty needle. He’s clean now. Not just from the virus-from the whole cycle. This drug didn’t just save his liver. It saved his soul.
Shanna Talley
November 6, 2025 AT 13:04It’s wild to think that something so simple could end a global health crisis. No magic bullets. Just smart science, patience, and the courage to make it affordable. We’ve proven that healing can be accessible. That’s the real win.
Samuel Wood
November 7, 2025 AT 03:21Let’s be honest-ledipasvir is just a glorified Band-Aid. We’re curing the virus but not addressing the root causes: poverty, needle sharing, lack of screening. You can’t treat a symptom and call it progress. True medicine is prevention. And we’re still failing at that.
ridar aeen
November 7, 2025 AT 14:36Actually, I think the real hero here is the WHO’s push for generics. Without them, this would’ve stayed a luxury for the rich. The fact that we’re even having this conversation now? That’s the revolution.
chantall meyer
November 9, 2025 AT 01:17Interesting how the West acts like they discovered this cure. Meanwhile, Indian manufacturers were making it for pennies while Americans were screaming about cost. The hypocrisy is exhausting.
Lorne Wellington
November 9, 2025 AT 23:18Remember when we used to say 'cure' like it was sci-fi? Now it’s just Tuesday. I’ve seen patients go from wheelchair to kayak in six months. This isn’t medicine-it’s a second chance. And we’re still not giving it to everyone who needs it. Shame.
Will RD
November 10, 2025 AT 02:49if you got hcv you deserved it. dont blame the drug for your bad choices
Jacqueline Anwar
November 11, 2025 AT 04:46While the clinical outcomes are undeniably impressive, the ethical implications of pharmaceutical pricing models during the initial rollout cannot be understated. This case serves as a textbook example of market failure in healthcare commodification.